The materials and test protocols used in the work are presented in the following sections.
Masks
The filtering materials of four non-medical masks were tested in this study. The masks were made from different fabrics produced by French manufacturers. Throughout this article, the masks will be referred to as A, B, C and D. The breathing performance was measured as the air permeability for a vacuum pressure of 100 Pa. The results for the four mask samples are Table 1. It is observed that the air permeability covers a large range from 100 to 300 L m−2 s−1.
Table 1 Permeability of 4 masks models used in this study.
Particle filtration efficiency (PFE) test protocol
The PFE protocol used in this study was developed by the French Defense Procurement Agency (DGA). The DGA is one of the agencies that is able to test the performance of non-medical masks. The PFE values evaluated in this study were obtained from the DGA reports provided to the manufacturers. The experimental setup consists of a test bench which allowed the generation and measurement of aerosols. Poly-disperse Holi particles were generated with a RBG 1000 PALAS. The Holi particles were generated with a median diameter of 1.1 µm (GSD of 1.6) and an average total concentration of 350 particles cm−3 (Fig. 1). A mask sample (cut out from full mask) was placed in a sample holder which were fabricated in-house and had an internal diameter of 44 mm.
Figure 1
Size distribution of Holi particles.
Two aerodynamic particle sizers (APS, model 3321; TSI Inc.) and two sample holders (one with mask sample and the other one without) were used to measure simultaneously aerosol number concentrations upstream and downstream of the sample holder.
The particle size considered for filtration efficiency is fixed at 3 µm as per the AFNOR SPEC S76-001. The PFE is calculated as:
$$PFE=1- frac{{C}_{down}}{{C}_{up}}* 100$$
where Cdown and Cup are the particle concentrations downstream and upstream the mask sample respectively; a correction factor was applied on concentrations ratio to take into account the systematic bias between the two measurement channels. The correction factor was calculated with data from a test without the sample on the sample holders. The PFE value is the average of three measurements of the same sample.
Bacterial filtration efficieny (BFE) test protocol
The BFE tests were performed by the accredited laboratory of the Centre Ingénierie et Santé (CIS) of Mines Saint-Etienne. The test protocol is compliant with the EN14683:2019 standard test method. The experimental set-up previously described in26 and27 is shown in Fig. 2.
Figure 2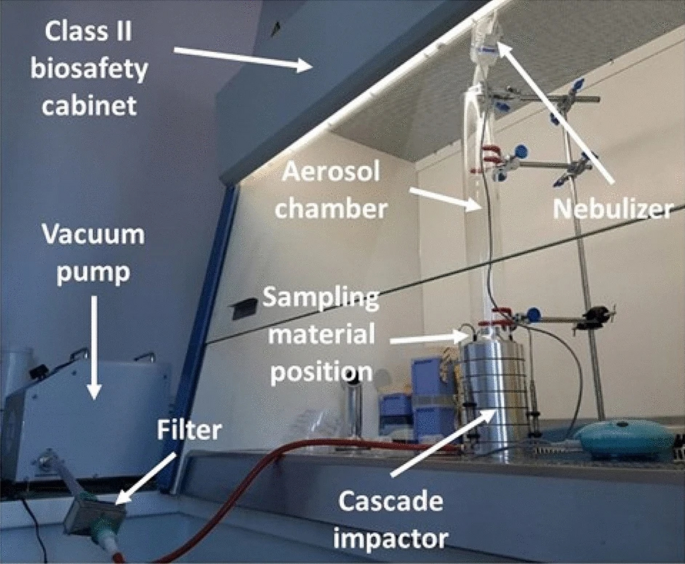
BFE experimental set-up developed by CIS of Mines Saint-Etienne.
Briefly, the mask sample was clamped between an aerosol chamber (glass tube 60 mm in external diameter and 60 mm long) and a six-stage viable aerosol cascade impactor (ACI) (Tisch Environmental, Cleaves, USA) requiring a flowrate of 28.3 L min−1. The 50% effective cut-off diameters (i.e., the particle diameters corresponding to 50% sampling efficiency) for each of the six stages when operating at 28.3 L min−1 are 7 µm (stage 1), 4.7 µm, 3.3 µm, 2.1 µm, 1.1 µm and 0.65 µm (stage 6).
Each sample measured at least 100 mm × 100 mm and the test area was therefore at least 49 cm2 as required by EN 14683. The tests were performed by putting the interior of the mask in contact with the aerosolized bacteria. Each sample was conditioned at 21 ± 5 °C and 85 ± 5% relative humidity for at least 4 h to reach atmospheric equilibrium prior to testing.
A bacterial culture of Staphylococcus aureus (ATCC 29213) was inoculated into 30 ml tryptic soy broth in an Erlenmeyer flask and incubated with mild shaking at a temperature of (37 ± 2) °C for (24 ± 2) h. The counts are expressed in Colony Forming Units (CFU). The culture medium was then diluted to obtain a concentration of approximately 5 × 105 CFU ml−1 for the tests. The inoculum was stored in a freezer at − 80 °C. The bacteriological inoculum was maintained on average between 1.7 × 103 CFU and 3.0 × 103 CFU as required in EN 14683. The EN 14683 also imposes a mean particle size (MPS) of 3.0 ± 0.3 µm. The bacterial challenge corresponds to the total CFU observed on the six positive-control dishes.
$$MPS= frac{left(P1* C1right)+left(P2* C2right)+left(P3 * C3right)+left(P4 * C4right)+left(P5* C5right)+left(P6 * C6right)}{C1+C2+C3+C4+C5+C6}$$
Px is the the 50% effective cut-off diameters of each of the six stages, Cx is the viable particle counts obtained from each of the six agar collection surfaces and x is the stage number (1–6).
The bacteriological inoculum was nebulized using an E-Flow mesh nebulizer (Pari GmbH, Starnberg, Germany) for 1 min and then the air flow in the cascade impactor was maintained for an additional minute (total test duration was 2 min). To evaluate the BFE of a mask, a series of eight successive measurements must be performed. First, a positive-control run was performed without a mask positioned between the cascade impactor and aerosol chamber. Next, five experiments were performed on test samples, changing the mask for each experiment and cleaning the experimental set-up to avoid bacterial contamination. A second positive control experiment was then performed. Finally, this cycle of eight consecutive experiments ended with a negative-control run in which air is passed, without adding bacteria, through the cascade impactor for 2 min (this served as a contamination control to verify that the bacteria deposited during the positive run and the test samples came only from the bioaerosol source).
In an effort to provide insight on the possible modernization of the standards on BFE analysis, Pourchez et al.26. showed that: (i) 90-mm disposable Petri dishes can be used instead of the 100-mm dishes supplied with the six-stage viable ACI and (ii) Automatic HD colony counters can be used to directly count viable particles on collection substrates without requiring the use of the positive-hole conversion table. In this work, 90-mm disposable Petri dishes were used. The petri dishes were removed and incubated at 37 ± 2 °C for 22 ± 2 h. The CFU were counted with an automatic colony counter Scan 1200 (Interscience).
The BFE is calculated as:
$$BFE= frac{C-T}{C * 100}$$
where C is the mean of the two positive runs of the total of the six plate counts, and T is the total of the six plate counts for each test sample. Five samples each are tested for Masks A and B, 4 samples for Mask C and 3 samples for Mask D.
Statistical analysis
Statistical analyses were performed with GraphPad Prism 8.4.2 (GraphPad Software, San Diego, CA, USA). A conformity analysis of the BFE to the 95% minimum value for Type I masks (EN 14683) was performed using the student t-test. In order to evaluate if there were any significant differences between the filtration efficiency results of the PFE test method and the BFE test method a Wilcoxon test was used. p-values < 0.05 were considered significant. Correlation coefficients for the comparison of filtration efficiency between the test methods were obtained using Excel.
[ad_2]
Originally Appeared Here